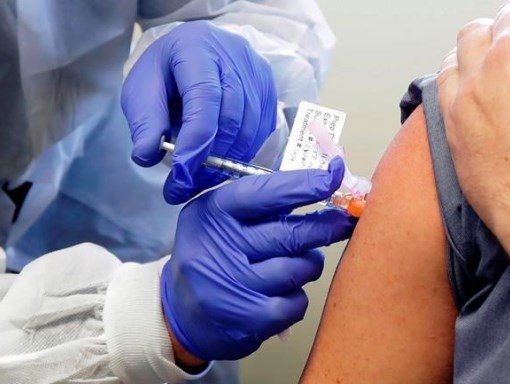
A two-shot COVID-19 vaccine developed by Pfizer and BioNTech is more than 90 per cent effective, according to data from an independent late-stage study released November 9.
The breakthrough vaccine could be available by the end of this month.
The U.S drug manufacturer and the German biotech firm released the report based on the first interim efficacy analysis conducted on November 8, 2020 by an external, independent Data Monitoring Committee (DMC) from the Phase 3 clinical study.
The data indicates “a vaccine efficacy rate above 90 per cent at 7 days after the second dose. This means that protection is achieved 28 days after the initiation of the vaccination, which consists of a two-dose schedule,” states a release from the two companies.
The DMC has not reported any serious safety concerns and recommends that the study continue to collect additional data as planned. The data will be discussed with regulatory authorities worldwide.
“Today is a great day for science and humanity. The first set of results from our Phase 3 COVID-19 vaccine trial provides the initial evidence of our vaccine’s ability to prevent COVID-19,” said Dr. Albert Bourla, Pfizer Chairman and CEO. “With today’s news, we are a significant step closer to providing people around the world with a much-needed breakthrough to help bring an end to this global health crisis.”
“The first interim analysis of our global Phase 3 study provides evidence that a vaccine may effectively prevent COVID-19. This is a victory for innovation, science and a global collaborative effort,” said Ugur Sahin, BioNTech co-founder and CEO. “When we embarked on this journey 10 months ago this is what we aspired to achieve. Especially today, while we are all in the midst of a second wave and many of us in lockdown, we appreciate even more how important this milestone is on our path towards ending this pandemic and for all of us to regain a sense of normality. We will continue to collect further data as the trial continues to enroll for a final analysis planned when a total of 164 confirmed COVID-19 cases have accrued. I would like to thank everyone who has contributed to make this important achievement possible.”
The Phase 3 clinical trial of BNT162b2 began on July 27 and has enrolled 43,538 participants to date, 38,955 of whom have received a second dose of the vaccine candidate as of November 8, 2020.
The 90 per cent success rate is well above the 50 percent effectiveness that the U.S. Food and Drug Administration requires for a coronavirus vaccine and far higher than the typical efficacy of the seasonal flu shot, which was only 29 percent effective in the 2018-2019 flu season, according to the U.S.-based Centers for Disease Control and Prevention (CDC).
The results suggest Pfizer’s two-stage vaccine works almost as well as the measles vaccine, which is about 93 percent effective with one dose and roughly 97 percent effective with two doses according to the CDC.
Pfizer said it plans to request the US Food and Drug Administration to approve the vaccine for emergency use by the end of November, if test results expected by the third week of November prove it is safe.
“Based on current projections we expect to produce globally up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021,” according to a Pfizer statement.
Canada had preordered the Pfizer-BioNTech vaccine in August, with a reservation of 20 million doses with the option to purchase more.